- The degree of hotness or coldness of a body or environment.
- A measure of the warmth or coldness of an object or substance with reference to some standard value.
- A measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale.
- A measure of the ability of a substance, or more generally of any physical system, to transfer heat energy to another physical system.
- Any of various standardized numerical measures of this ability, such as the Kelvin, Fahrenheit, and Celsius scale
As mentioned, the first two bullet points have rather obvious meanings. The third bullet point was the topic of
the previous page in this lesson. The fifth bullet point was the definition that we started with as we discussed temperature and the operation of thermometers; it was the topic of
the second page in this lesson. That leaves us with the fourth bullet point - defining temperature in terms of the ability of a substance to transfer heat to another substance. This part of Lesson 1 is devoted to understanding how the relative temperature of two objects affects the direction that heat is transferred between the two objects.
What is Heat?
Consider a very hot mug of coffee on the countertop of your kitchen. For discussion purposes, we will say that the cup of coffee has a temperature of 80°C and that the surroundings (countertop, air in the kitchen, etc.) has a temperature of 26°C. What do you suppose will happen in this situation? I suspect that you know that the cup of coffee will gradually cool down over time. At 80°C, you wouldn't dare drink the coffee.
Even the coffee mug will likely be too hot to touch. But over time, both the coffee mug and the coffee will cool down. Soon it will be at a drinkable temperature. And if you resist the temptation to drink the coffee, it will eventually reach room temperature. The coffee cools from 80°C to about 26°C. So what is happening over the course of time to cause the coffee to cool down? The answer to this question can be both
macroscopic and
particulate in nature.
On the macroscopic level, we would say that the coffee and the mug are transferring heat to the surroundings. This transfer of heat occurs from the hot coffee and hot mug to the surrounding air. The fact that the coffee lowers its temperature is a sign that the average kinetic energy of its particles is decreasing. The coffee is losing energy. The mug is also lowering its temperature; the average kinetic energy of its particles is also decreasing. The mug is also losing energy. The energy that is lost by the coffee and the mug is being transferred to the colder surroundings. We refer to this transfer of energy from the coffee and the mug to the surrounding air and countertop as heat. In this sense,
heat is simply the transfer of energy from a hot object to a colder object.
Now let's consider a different scenario - that of a cold can of pop placed on the same kitchen counter. For discussion purposes, we will say that the pop and the can which contains it has a temperature of 5°C and that the surroundings (countertop, air in the kitchen, etc.) has a temperature of 26°C. What will happen to the cold can of pop over the course of time? Once more, I suspect that you know the answer. The cold pop and the container will both warm up to room temperature. But what is happening to cause these colder-than-room-temperature objects to increase their temperature? Is the cold escaping from the pop and its container? No! There is no such thing as
the cold escaping or
leaking. Rather, our explanation is very similar to the explanation used to explain why the coffee cools down. There is a heat transfer.

Over time, the pop and the container increase their temperature. The temperature rises from 5°C to nearly 26°C. This increase in temperature is a sign that the average kinetic energy of the particles within the pop and the container is increasing. In order for the particles within the pop and the container to increase their kinetic energy, they must be gaining energy from somewhere. But from where? Energy is being transferred from the surroundings (countertop, air in the kitchen, etc.) in the form of heat. Just as in the case of the cooling coffee mug, energy is being transferred from the higher temperature objects to the lower temperature object. Once more, this is known as heat - the transfer of energy from the higher temperature object to a lower temperature object.
Another Definition of Temperature
Both of these scenarios could be summarized by two simple statements. An object decreases its temperature by releasing energy in the form of heat to its surroundings. And an object increases its temperature by gaining energy in the form of heat from its surroundings. Both the warming up and the cooling down of objects works in the same way - by heat transfer from the higher temperature object to the lower temperature object. So now we can meaningfully re-state the definition of temperature. Temperature is a measure of the ability of a substance, or more generally of any physical system, to transfer heat energy to another physical system. The higher the temperature of an object is, the greater the tendency of that object to transfer heat. The lower the temperature of an object is, the greater the tendency of that object to be on the receiving end of the heat transfer.
But perhaps you have been asking: what happens to the temperature of surroundings? Do the countertop and the air in the kitchen increase their temperature when the mug and the coffee cool down? And do the countertop and the air in the kitchen decrease its temperature when the can and its pop warm up? The answer is a resounding Yes! The proof? Just touch the countertop - it should feel cooler or warmer than before the coffee mug or pop can were placed on the countertop. But what about the air in the kitchen? Now that's a little more difficult to present a convincing proof of. The fact that the volume of air in the room is so large and that the energy quickly diffuses away from the surface of the mug means that the temperature change of the air in the kitchen will be abnormally small. In fact, it will be
negligibly small. There would have to be a lot more heat transfer before there is a noticeable temperature change.
Thermal Equilibrium
In the discussion of the cooling of the coffee mug, the countertop and the air in the kitchen were referred to as the surroundings. It is common in physics discussions of this type to use a mental framework of a system and the surroundings. The coffee mug (and the coffee) would be regarded as the system and everything else in the universe would be regarded as the surroundings. To keep it simple, we often narrow the scope of the surroundings from the rest of the universe to simply those objects that are immediately surrounding the system. This approach of analyzing a situation in terms of system and surroundings is so useful that we will adopt the approach for the rest of this chapter and the next.
Now let's imagine a third situation. Suppose that a small metal cup of hot water is placed inside of a larger Styrofoam cup of cold water. Let's suppose that the temperature of the hot water is initially 70°C and that the temperature of the cold water in the outer cup is initially 5°C. And let's suppose that both cups are equipped with thermometers (or temperature probes) that measure the temperature of the water in each cup over the course of time. What do you suppose will happen? Before you read on, think about the question and commit to some form of answer. When the cold water is done warming and the hot water is done cooling, will their temperatures be the same or different? Will the cold water warm up to a lower temperature than the temperature that the hot water cools down to? Or as the warming and cooling occurs, will their temperatures
cross each other?
Fortunately, this is an experiment that can be done and in fact has been done on many occasions. The graph below is a typical representation of the results.
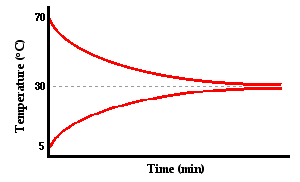
As you can see from the graph, the hot water cooled down to approximately 30°C and the cold water warmed up to approximately the same temperature. Heat is transferred from the high temperature object (inner can of hot water) to the low temperature object (outer can of cold water). If we designate the inner cup of hot water as
the system, then we can say that there is a flow of heat from
the system to the
surroundings. As long as there is a temperature difference between the system and the surroundings, there is a heat flow between them. The heat flow is more rapid at first as depicted by the steeper slopes of the lines. Over time, the temperature difference between system and surroundings decreases and the rate of heat transfer decreases. This is denoted by the gentler slope of the two lines. (Detailed information about rates of heat transfer will be discussed
later in this lesson.) Eventually, the system and the surroundings reach the same temperature and the heat transfer ceases. It is at this point, that the two objects are said to have reached
thermal equilibrium.
The Zeroeth Law of Thermodynamics
In our
chapter on electric circuits, we learned that a difference in electric potential between two locations causes a flow of charge along a conducting path between those locations. As long as an
electric potential difference is maintained, a flow of charge will exist. Now in this chapter we learn a similar principle related to the flow of heat. A temperature difference between two locations will cause a flow of heat along a (thermally) conducting path between those two locations. As long as the temperature difference is maintained, a flow of heat will occur. This flow of heat continues until the two objects reach the same temperature. Once their temperatures become equal, they are said to be at thermal equilibrium and the flow of heat no longer takes place.
This principle is sometimes referred to as the
zeroeth law of thermodynamics. This principle became formalized into a law after the first, second and third laws of thermodynamics had already been
discovered. But because the law seemed more fundamental than the previously discovered three, it was titled the
zeroeth law. All objects are governed by this law - this tendency towards thermal equilibrium. It represents a daily challenge for those who wish to control the temperature of their bodies, their food, their drinks and their homes. We use ice and insulation to try to keep our cold drinks cold and we use insulation and ongoing pulses of microwave energy to keep our hot drinks hot. We equip our vehicles, our homes and our office buildings equipped with air conditioners and fans in order to keep them cool during the warm summer months. And we equip these same vehicles and buildings with furnaces and heaters in order to keep them warm during the cold winter months. Whenever any of these systems are at a different temperature as the surroundings and not perfectly insulated from the surroundings (an ideal situation), heat will flow. This heat flow will continue until the system and surroundings have achieved equal temperatures. Because these systems have a considerably smaller volume than the surroundings, there will be a more noticeable and substantial change in temperature of these systems.
The Caloric Theory
Scientists have long pondered the nature of heat. Well into the mid-19th century, the most accepted notion of heat was one that associated it with a fluid known as caloric. Noted chemist Antoine Lavoisier reasoned that there were two forms of caloric - the kind that was latent or stored in combustible materials and the kind that was sensible and observable through a temperature change. For Lavoisier and his followers, the burning of fuel resulted in the release of this latent heat to the surroundings where it was observed to cause a temperature change of the surroundings. To Lavoisier and his followers, the heat was always present - either in latent form or in sensible form. If a hot kettle of water cooled down to room temperature, it was explained by the flow of caloric from the hot water to the surroundings.

According to caloric theory, heat was
material in nature. It was a physical substance. It was
stuff. Like all stuff in Lavoisier's world, caloric was a conserved substance. Similar to our modern view of heat, the calorist view was that if caloric was released by one object, then it was gained by another object. The total amount of caloric never changed; it was simply transferred from one object to another and transformed from one type (latent) to another type (sensible). But unlike our modern view of heat, caloric was an actual physical substance - a fluid that could flow from one object to another. And unlike our modern view, heat was always present in one form or another. Finally, in the modern view, heat is present only when there is an energy transfer. It is senseless to speak of the heat as still existing once the two objects have come to thermal equilibrium. Heat is not something contained in an object; rather it is something transferred between objects. The heat no longer exists when the transfer ceases.
The Fall of Caloric Theory
While there were always alternatives to the caloric theory, it was the most accepted view up until the mid 19th century. One of the first challenges to the caloric theory was from Anglo-American scientist Benjamin Thompson (a.k.a., Count Rumford). Thompson was one of the primary scientists appointed to the task of boring out the barrels of cannons for the British government. Thompson was amazed by the high temperatures reached by the cannons and by the shavings that were shed from the cannons during the boring process. In one experiment, he immersed the cannon in a tank of water during the boring process and observed that the heat generated by the boring process was capable of boiling the surrounding water within a few hours. Thompson demonstrated that this heat generation occurred in the absence of any chemical or physical change in the cannon's composition. He attributed the generation of heat to friction between the cannon and the boring tool and argued that it could not have been the result of the flow of fluid into the water. Thompson published a paper in 1798 that challenged the view that heat was a fluid that was conserved. He advocated a mechanical view of heat, suggesting that its origin was related to the motion of atoms and not the transfer of a fluid.
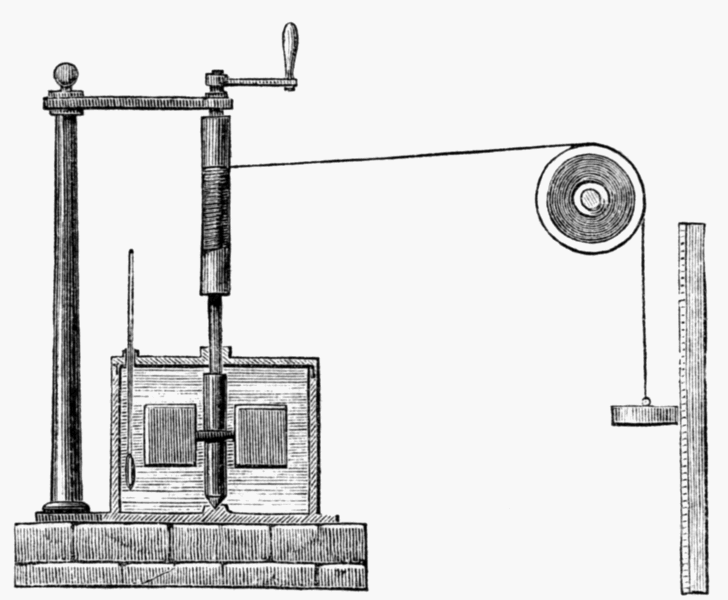
English physicist James Prescott Joule took up where Thompson left off, delivering several fateful blows to the caloric theory through a collection of experiments. Joule, for whom the standard metric unit of energy is now named, performed experiments in which he experimentally related the amount of mechanical work to the amount of heat transferred from the mechanical system. In one experiment, Joule allowed falling weights to turn a paddle wheel that was submerged in a reservoir of water. A drawing of the apparatus is depicted at the right (from Wikimedia;
public domain). The falling weights did work on the paddle wheel, which in turn heated the water. Joule measured both the amount of mechanical work done and the amount of heat gained by the water. Similar experiments demonstrating that heat could be generated by an electric current dealt a further blow to the thought that heat was a fluid that was contained within substances and was always conserved.
As we will learn in great detail in the next chapter, objects possess internal energy. In chemical reactions, a portion of this energy can be released to the surroundings in the form of heat. However, this internal energy is not a material substance or a fluid contained by the object. It is simply the potential energy stored in the bonds that hold particles within the object together. Heat or thermal energy is the form this energy possesses when it is being transferred between
systems and
surroundings. There is nothing material about heat. It is neither a substance nor a fluid that is conserved. Heat is a form of energy that can be transferred from one object to another or even created at the expense of the loss of other forms of energy.
To review, temperature is a measure of the ability of a substance, or more generally of any physical system, to transfer heat energy to another physical system. If two objects - or if a system and its surroundings - have a different temperature, then they have a different ability to transfer heat. Over time, there will be a flow of energy from the hotter object to the cooler object. This flow of energy is referred to as heat. The heat flow causes the hotter object to cool down and the colder object to warm up. The flow of heat will continue until they reach the same temperature. At this point, the two objects have established a thermal equilibrium with each other.
In the
next part of this lesson, we will explore the mechanism of heat transfer. We will look at the various methods by which heat can be transferred from object to object or even from one location within an object to another. We will learn that the macroscopic can be explained in terms of the microscopic.







 On the macroscopic level, we would say that the coffee and the mug are transferring heat to the surroundings. This transfer of heat occurs from the hot coffee and hot mug to the surrounding air. The fact that the coffee lowers its temperature is a sign that the average kinetic energy of its particles is decreasing. The coffee is losing energy. The mug is also lowering its temperature; the average kinetic energy of its particles is also decreasing. The mug is also losing energy. The energy that is lost by the coffee and the mug is being transferred to the colder surroundings. We refer to this transfer of energy from the coffee and the mug to the surrounding air and countertop as heat. In this sense,
On the macroscopic level, we would say that the coffee and the mug are transferring heat to the surroundings. This transfer of heat occurs from the hot coffee and hot mug to the surrounding air. The fact that the coffee lowers its temperature is a sign that the average kinetic energy of its particles is decreasing. The coffee is losing energy. The mug is also lowering its temperature; the average kinetic energy of its particles is also decreasing. The mug is also losing energy. The energy that is lost by the coffee and the mug is being transferred to the colder surroundings. We refer to this transfer of energy from the coffee and the mug to the surrounding air and countertop as heat. In this sense,  Over time, the pop and the container increase their temperature. The temperature rises from 5°C to nearly 26°C. This increase in temperature is a sign that the average kinetic energy of the particles within the pop and the container is increasing. In order for the particles within the pop and the container to increase their kinetic energy, they must be gaining energy from somewhere. But from where? Energy is being transferred from the surroundings (countertop, air in the kitchen, etc.) in the form of heat. Just as in the case of the cooling coffee mug, energy is being transferred from the higher temperature objects to the lower temperature object. Once more, this is known as heat - the transfer of energy from the higher temperature object to a lower temperature object.
Over time, the pop and the container increase their temperature. The temperature rises from 5°C to nearly 26°C. This increase in temperature is a sign that the average kinetic energy of the particles within the pop and the container is increasing. In order for the particles within the pop and the container to increase their kinetic energy, they must be gaining energy from somewhere. But from where? Energy is being transferred from the surroundings (countertop, air in the kitchen, etc.) in the form of heat. Just as in the case of the cooling coffee mug, energy is being transferred from the higher temperature objects to the lower temperature object. Once more, this is known as heat - the transfer of energy from the higher temperature object to a lower temperature object.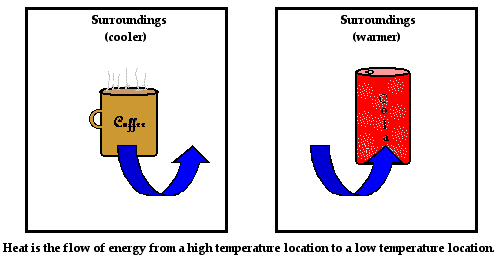


 According to caloric theory, heat was material in nature. It was a physical substance. It was stuff. Like all stuff in Lavoisier's world, caloric was a conserved substance. Similar to our modern view of heat, the calorist view was that if caloric was released by one object, then it was gained by another object. The total amount of caloric never changed; it was simply transferred from one object to another and transformed from one type (latent) to another type (sensible). But unlike our modern view of heat, caloric was an actual physical substance - a fluid that could flow from one object to another. And unlike our modern view, heat was always present in one form or another. Finally, in the modern view, heat is present only when there is an energy transfer. It is senseless to speak of the heat as still existing once the two objects have come to thermal equilibrium. Heat is not something contained in an object; rather it is something transferred between objects. The heat no longer exists when the transfer ceases.
According to caloric theory, heat was material in nature. It was a physical substance. It was stuff. Like all stuff in Lavoisier's world, caloric was a conserved substance. Similar to our modern view of heat, the calorist view was that if caloric was released by one object, then it was gained by another object. The total amount of caloric never changed; it was simply transferred from one object to another and transformed from one type (latent) to another type (sensible). But unlike our modern view of heat, caloric was an actual physical substance - a fluid that could flow from one object to another. And unlike our modern view, heat was always present in one form or another. Finally, in the modern view, heat is present only when there is an energy transfer. It is senseless to speak of the heat as still existing once the two objects have come to thermal equilibrium. Heat is not something contained in an object; rather it is something transferred between objects. The heat no longer exists when the transfer ceases. English physicist James Prescott Joule took up where Thompson left off, delivering several fateful blows to the caloric theory through a collection of experiments. Joule, for whom the standard metric unit of energy is now named, performed experiments in which he experimentally related the amount of mechanical work to the amount of heat transferred from the mechanical system. In one experiment, Joule allowed falling weights to turn a paddle wheel that was submerged in a reservoir of water. A drawing of the apparatus is depicted at the right (from Wikimedia;
English physicist James Prescott Joule took up where Thompson left off, delivering several fateful blows to the caloric theory through a collection of experiments. Joule, for whom the standard metric unit of energy is now named, performed experiments in which he experimentally related the amount of mechanical work to the amount of heat transferred from the mechanical system. In one experiment, Joule allowed falling weights to turn a paddle wheel that was submerged in a reservoir of water. A drawing of the apparatus is depicted at the right (from Wikimedia; 


